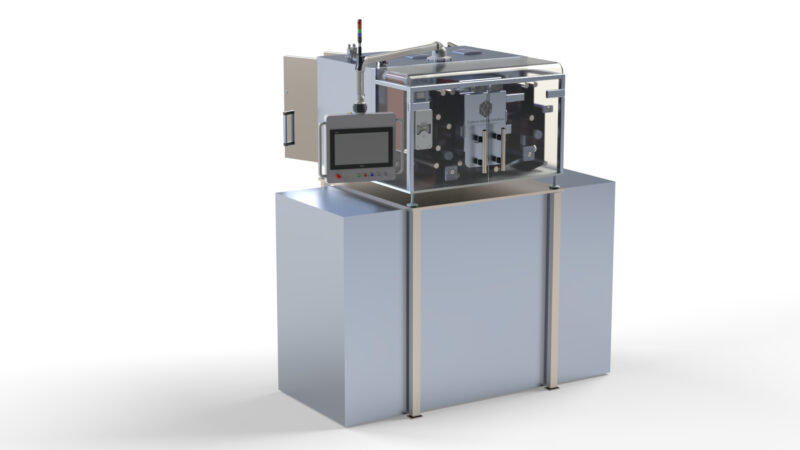
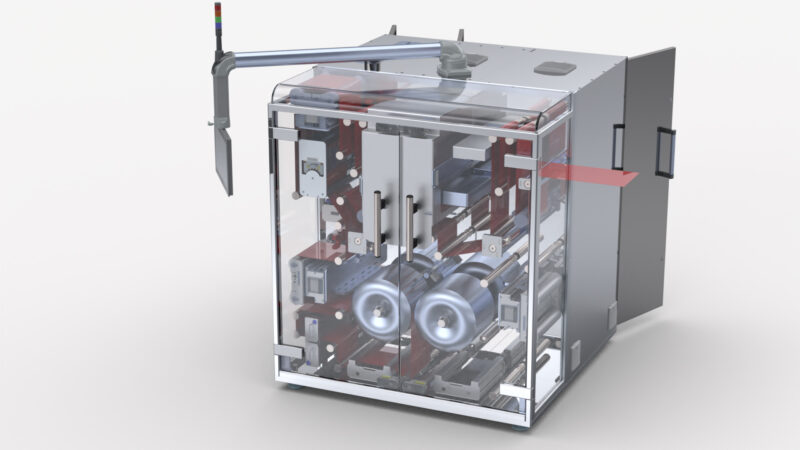
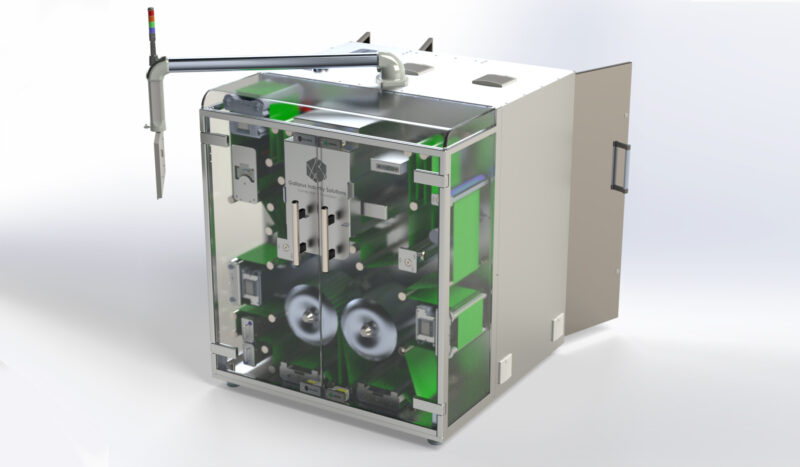
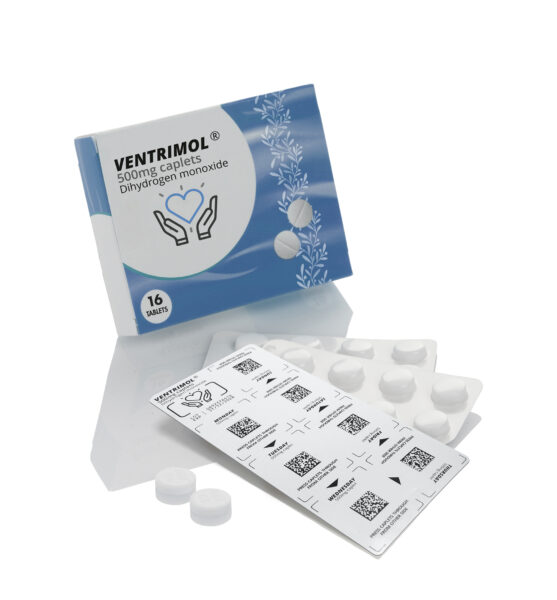
Domino Printing Sciences (Domino) is pleased to announce the launch of the new K600G – an innovative new blister foil and web digital printing solution for product-level serialisation in pharmaceutical applications.
Developed in collaboration with pharmaceutical industry innovator Gallarus, and with engagement from life science industry experts SeaVision, the K600G is a ground-breaking, high-resolution, digital printing solution. The result of successful teamwork between market leaders in digital print and life science experts, the K600G promises to meet the needs of pharmaceutical manufacturers now and in the future.
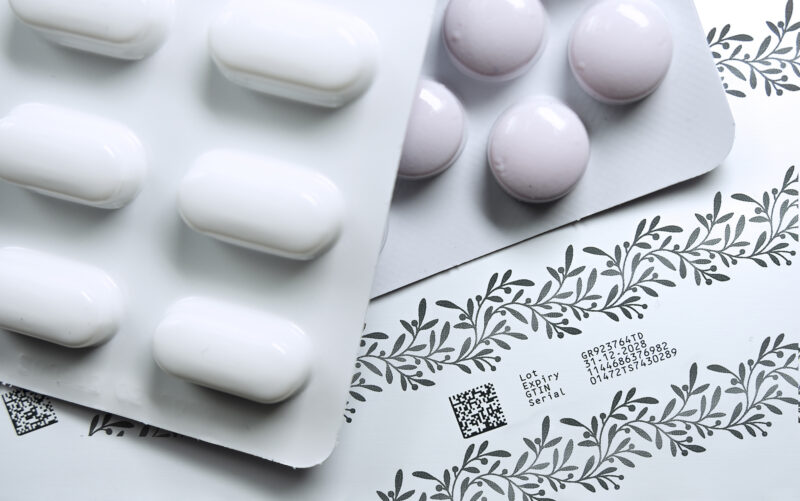
“Over the last couple of years serialisation at pack level has become a global requirement in the pharmaceutical industry,” says Craig Stobie, Director of Global Sector Development at Domino. “In the future, it is likely that serialisation of individual pockets of a blister pack will become the new standard, in order to further enhance patient safety and reduce medical errors.
“The K600G has been developed to provide manufacturers with an on-site solution for variable data printing, which includes coding at the item level, to meet these future requirements,” he continues.
Using digital printing technology to customise product packaging within manufacturing chains adds a degree of agility that allows pharmaceutical manufacturers to react more easily to legislative changes or variations in product labelling requirements, as it reduces reliance on external packaging providers.
The new K600G includes a range of solutions that have been developed for different installation types, including off-line, near-line, top-of-line, sealed-blister-coding for printing directly on formed blister packs, and an integrated-line version developed to meet the needs of OEMs.
“Digital printing and flexible supply chains are beginning to play a much greater role in pharmaceutical manufacturing – and so the K600G is a necessary investment to keep up with future legislation and market trends,” says Volker Watzke, EU Medical Devices Sector Manager at Domino.
“As Domino customers, pharmaceutical manufacturers utilising the K600G solution also have the benefit of our global experts, who are always ready to support them when they need us,” he adds. “We want to ensure that our customers are always in control of their lines.”
When it comes to printing performance, the K600G achieves high-quality, reliable printing across a range of substrates – the printing module has a native resolution of 600dpi and excellent greyscale capability. Based on Domino’s market-leading piezo drop-on-demand ink jet technology, the printer is also capable of building up imagery using multiple different drop sizes – this improves image quality and gives manufacturers more control over their ink consumption.
“In pharmaceutical manufacturing, code quality and legibility are of key importance, and, owing to the cost of unplanned downtime, it is also imperative that machines are kept functioning at optimal performance,” says Bart Vansteenkiste, Global Sector Manager – Life Sciences at Domino. “The K600G was developed with this level of reliability and durability in mind with automated maintenance systems to reduce operator intervention and to keep the system running at optimal performance.”
In order to achieve the highest levels of efficiency, the system can also be supplied with a unique AI software, that collects, analyses, and learns from factory data to provide intelligent manufacturing solutions.
“With the K600G, we have combined the key principles of Industry 4.0 to provide a solution that addresses the evolving life science environment and production challenges facing pharmaceutical manufacturers,” says John McKeon, CEO of Gallarus. “The new solution delivers exponential benefits in the form of ROI, cost-saving, quality enhancement, and OEE efficiencies.”
The K600G is capable of printing at speeds of up to 75 metres per minute and print widths range from a single print module, covering 108mm (4.25”), up to seven dual print modules with a combined width of 782mm (30.81”). The smart i-Tech StitchLink micro-motor controller technology enables accurate print head alignment and image stitching to achieve seamless printing across a full web print width.
With its revolutionary i-Tech CleanCap, the K600G cleans the print heads when not in operation, leaving them wiped and capped, ready for the next use. This removes the need for daily print head cleaning, protects the print heads from blockage and damage, and reduces the need for maintenance. i-Tech ActiFlow ensures the ink is always moving around the print head. This degasses the ink, preventing the formation of air bubbles that can impact nozzle performance and increase the risk of product rejects.
“When we set out to develop the K600G we wanted to develop a future-proof solution that would meet the current needs of pharmaceutical manufacturers, and allow them to adapt more easily to changing market trends and legislative requirements,” says Watzke. “The result is a strong and reliable solution that helps to ensure compliance and patient safety while allowing manufacturers to remain agile. When it comes to pharmaceutical manufacturing, the K600G is the solution of choice, now, and in the future,” he concludes.
For more information on the K600G please visit https://bit.ly/2HwRcBE